Key difference allylic vs vinylic carbons functional groups are very important in understanding the different physical and chemical properties of organic molecules the terms allylic and vinyl carbons indicate whether the carbon atom is bonded directly or indirectly to a double bond in a molecule.
Is allylic carbon more acidic than vinyl carbon.
The key difference between allylic and vinylic carbon is that allylic carbon is the carbon.
Unlike vinyl group the allylic carbon atom is sp 3 hybridized as it bonded with ch ch 2.
The conjugate base anion is alkylated by allyl bromide.
An allylic carbon is one that is directly attached to a pi bond.
Do not confuse an allylic group with a vinyl group.
The general formula for allyl is r ch 2 ch ch 2 in which the asterisk carbon atom is an allylic carbon atom.
The key difference between these two structural components is the number of carbon and hydrogen atoms.
Allyl group holds three carbon atoms and five hydrogen atoms which gets attached to any other group of atoms through ch 2 group.
See also allylic hydrogen.
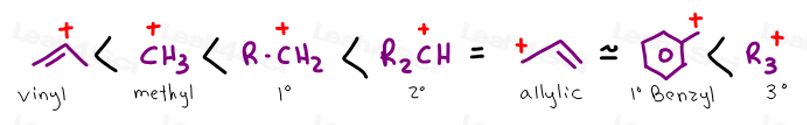
A vinyl carbocation has a positive charge on the same carbon as the double bond.
This heightened reactivity has many practical consequences.
The allylic carbon is bonded to a carbon atom which is doubly bonded to another carbon atom.
An allylic carbon is a carbon atom bonded to a carbon atom that in turn is doubly bonded to another carbon atom.
Both groups own a double bond between two carbon atoms where all the other atoms are bonded through single bonds.
The allylic carbon atom is more reactive than normal.
Allyl indicates a functional group with structural formula h 2 c ch ch 2 r where r is the rest of the molecule it consists of methylene bridge ch 2 in between the vinyl group ch ch 2 and the rest of the molecule therefore allyl group contains sp 2 hybridized vinyl carbon atoms and sp 3 hybridized allyl carbon atom.
It is one of the functional groups in organic chemistry which has the general molecular formula rch 2 ch ch 2.
An allylic system has a minimum of 3 carbons.
Allyl groups have three carbon atoms and five hydrogen atoms.
Key difference allyl vs vinyl both allyl and vinyl groups have slightly similar structures with a small variation.
C a hydrogen on the central carbon is more acidic than an acetylenic hydrogen because the conjugate base anion resulting from removal of the central hydrogen is both allylic and propargylic and is therefore doubly resonance stabilized.
The allylic position is also like a vinylic position.
A site adjacent to the unsaturated carbon atom is called the allylic position or allylic site a group attached at this site is sometimes described as allylic thus ch 2 chch 2 oh has an allylic hydroxyl group allylic c h bonds are about 15 weaker than the c h bonds in ordinary sp 3 carbon centers and are thus more reactive.